
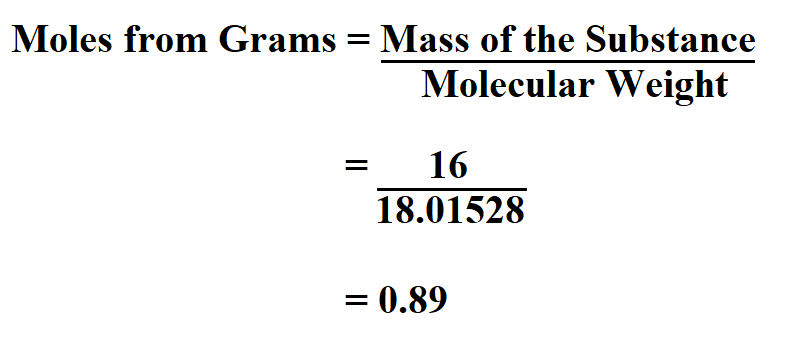
Since atoms are too small to count, we must weigh out a mole of atoms. It just demonstrates that mole of anything = 6.0221367E 23 and we can measure out a mole of something by counting it or by weighing it out. In order to determine the weight of one mole of bananas, one would have to get an average weight of a banana and multiply that by 6.0221367E 23 then we could weigh out that weight of bananas and presto, we would have a mole of bananas. A mole of any molecule = the molecular mass of that molecule expressed in grams. Since magnesium has an atomic mass of 24, one mole of magnesium weighs 24 grams and contains 6.0221367E 23 atoms of magnesium. It has been established that 1 mole of any element = the atomic mass of that element expressed in grams.

Remember, however that 1 mole of carbon-12 = 12 grams = 6.0221367E 23 atoms. Obviously it would be impossible to count out 6.0221367E 23 atoms. (Aren't you glad they don't sell bananas by the mole?) Examples: 1 mole of carbon = 6.0221367E 23 carbon atoms 1 mole of bananas = 6.0221367E 23 bananas One mole of anything, however, contains 6.0221367E 23 of that object. Remember that carbon-12 has an atomic mass of 12 (six neutrons and six protons).

By definition, in modern chemistry, one mole represents the number of carbon atoms in exactly 12 grams of carbon 12 isotope. The mole also called mol is the basic unit of measurement in chemistry. In order to grasp the concept of molar mass calculations it is important to understand the molar unit.


 0 kommentar(er)
0 kommentar(er)
